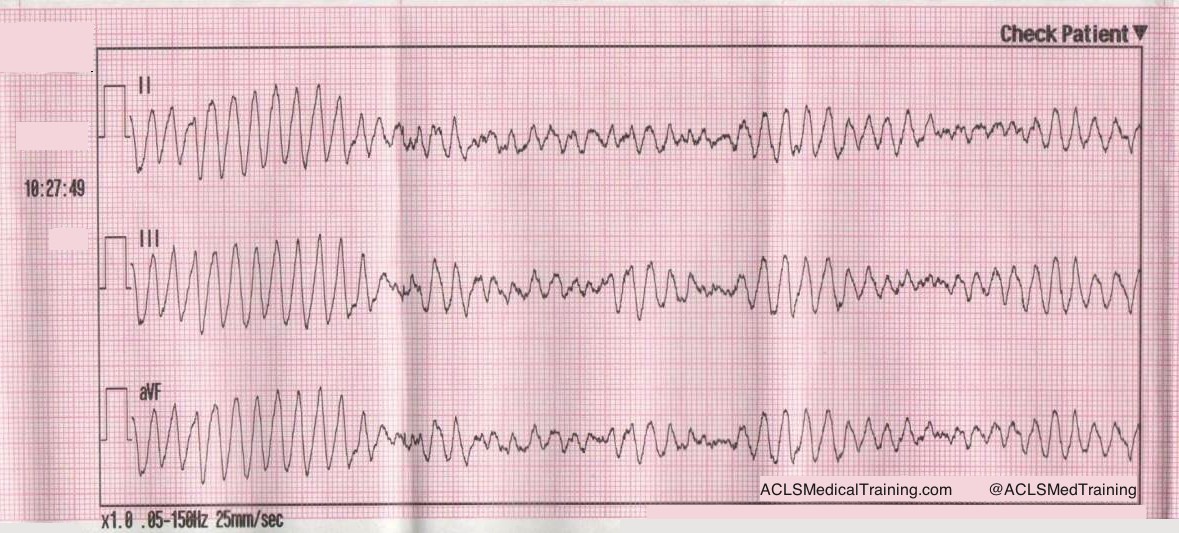
A 75 year old male experienced a syncopal episode. The event was witnessed by family members who contacted 9-1-1. On arrival of EMS personnel the patient appears acutely ill. He is pale, diaphoretic and cool to touch. He states that he is feeling lightheaded and weak.
Medical History
- Hypertension
- Hyperlipidemia
- Gout
- Bilateral knee replacement
- Left bundle branch block
The family reports the patient is seeing a cardiologist and is scheduled for pacemaker implantation in 3 weeks due to previous episodes of symptomatic bradycardia.
Medications
- Zocor (Simvastatin)
- Lopressor (Metoprolol)
- Aloprim (Allopurinol)
- Multi-vitamins
Vital Signs
- RR: 20
- HR: 20
- BP: 80/48 mm Hg
- SpO2: 93% on room air
- Capillary blood glucose: 118 mg/dL
Breath sounds are clear bilaterally.
The patient is placed on O2 via nasal cannula at 2 LPM with ETCO2 of 16 mmHg.
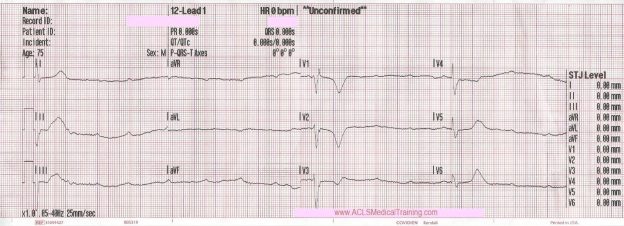
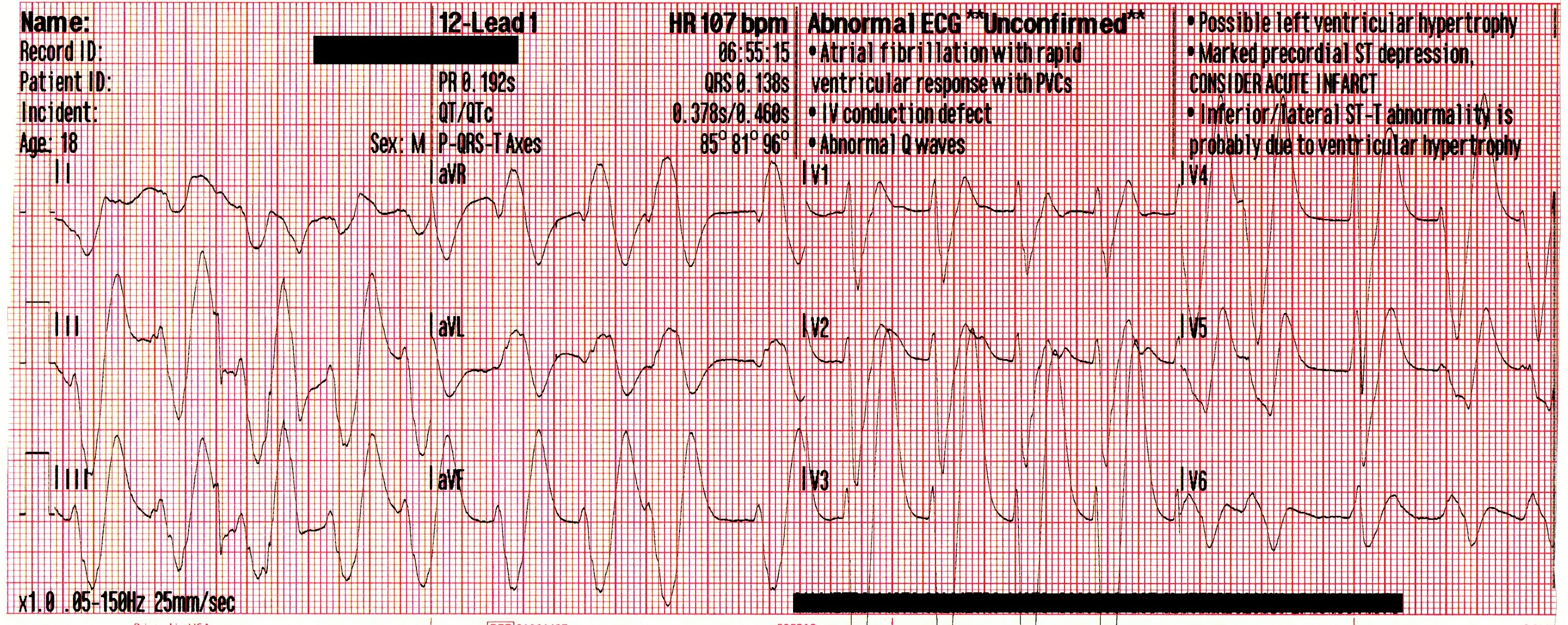
Cardiac monitoring is established and the following 12 lead ECG is obtained.
When considering bradycardia management, 3 initial questions should be answered:
- Is the patient bradycardic?
- Is the patient symptomatic?
- Is the patient symptomatic from the bradycardia?
If all of these have been answered with a YES, determine the patient’s hemodynamic status.
Signs of hemodynamically instability:
- Altered mental status
- Hypotension
- Ischemic chest pain
- Signs of hypoperfusion
- Acute pulmonary edema
Based on these criteria this patient is clearly unstable.
Defibrillation pads are placed as a precaution, IV access is obtained, and 250 ml of normal saline is administered en route to the Emergency Department which is 4 minutes away from the scene.
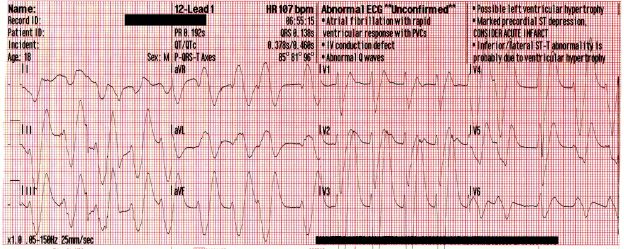
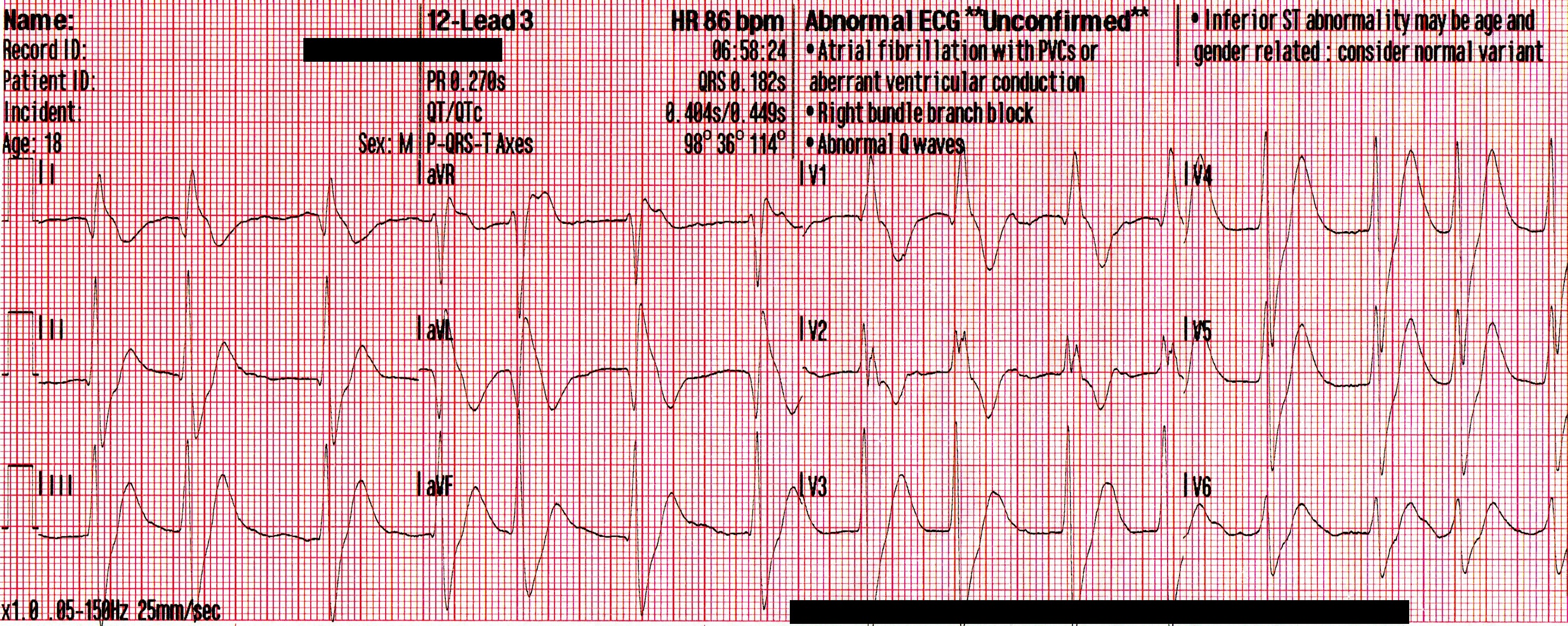
Upon arrival blood samples are obtained and the following 12 lead ECG is obtained.
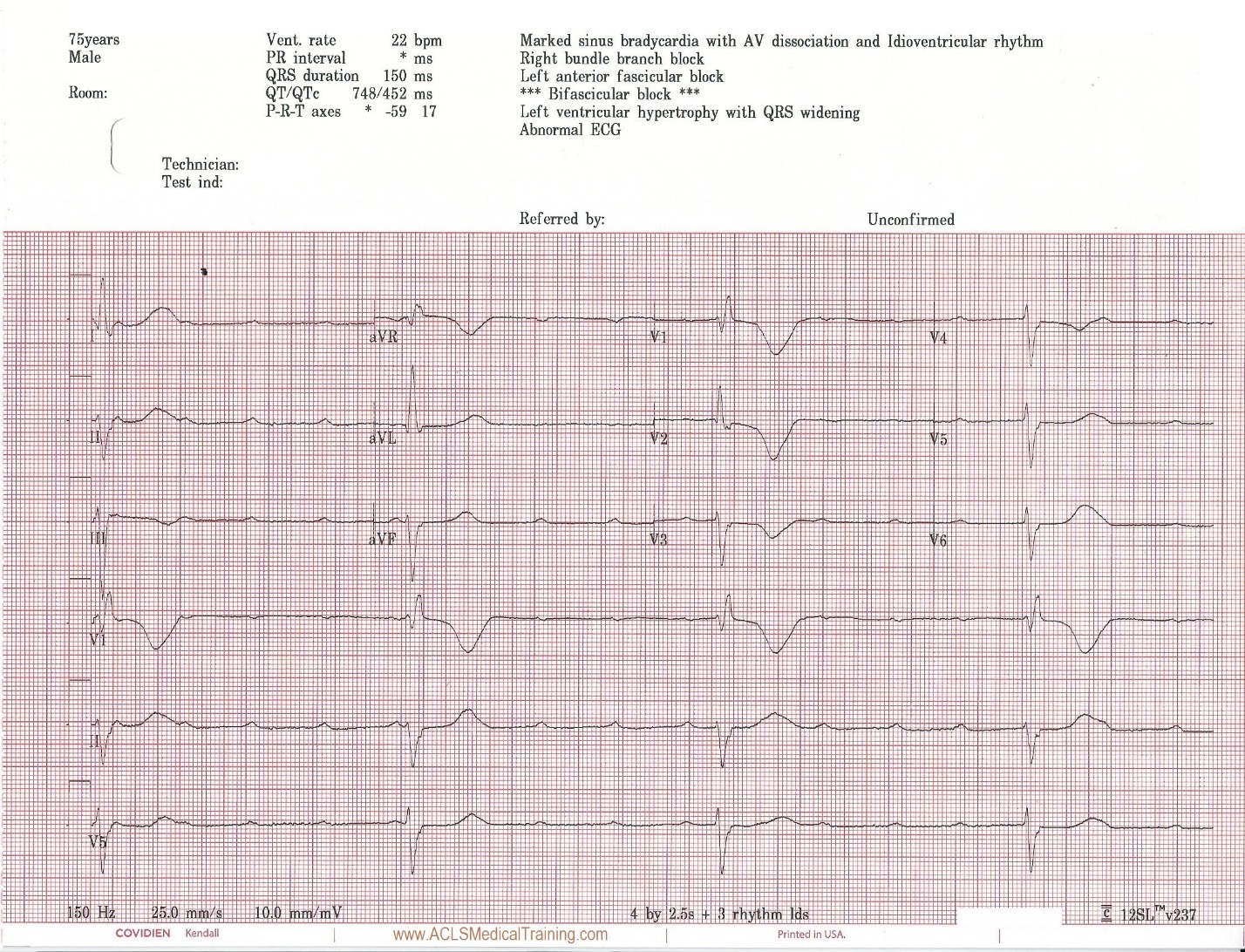
3rd Degree AV Block. The escape rhythm shows a wide QRS with bifascicular morphology (RBBB morphology with left axis deviation). It is likely a ventricular in origin.
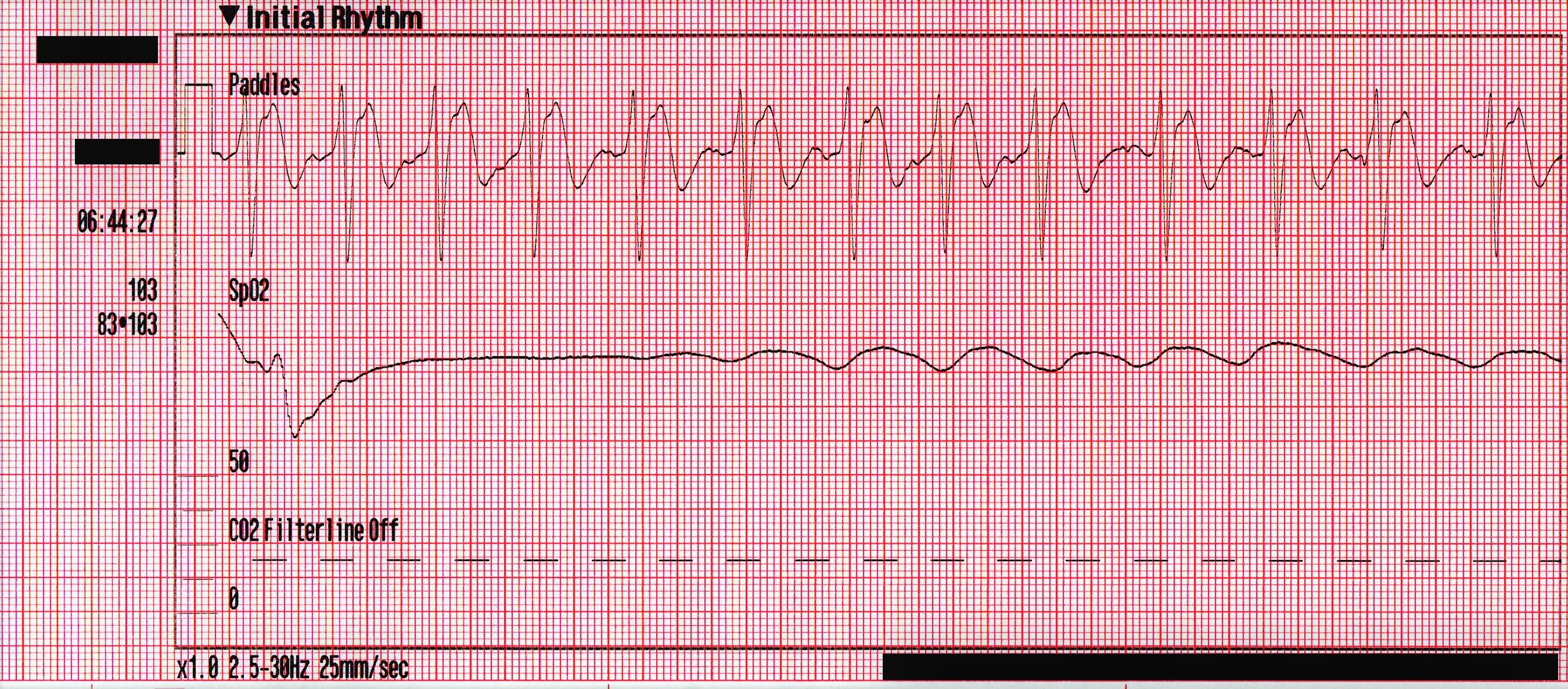
The patient’s level of consciousness deteriorates and he responds only to painful stimuli.
0.5 mg atropine is administered rapid IV push followed by 10 ml saline flush.
After 1 minute transcutaneous pacing is initiated with no electrical capture up to 90 mA. Transcutaneous pacing is discontinued by the arriving cardiologist who requests vasopressors.
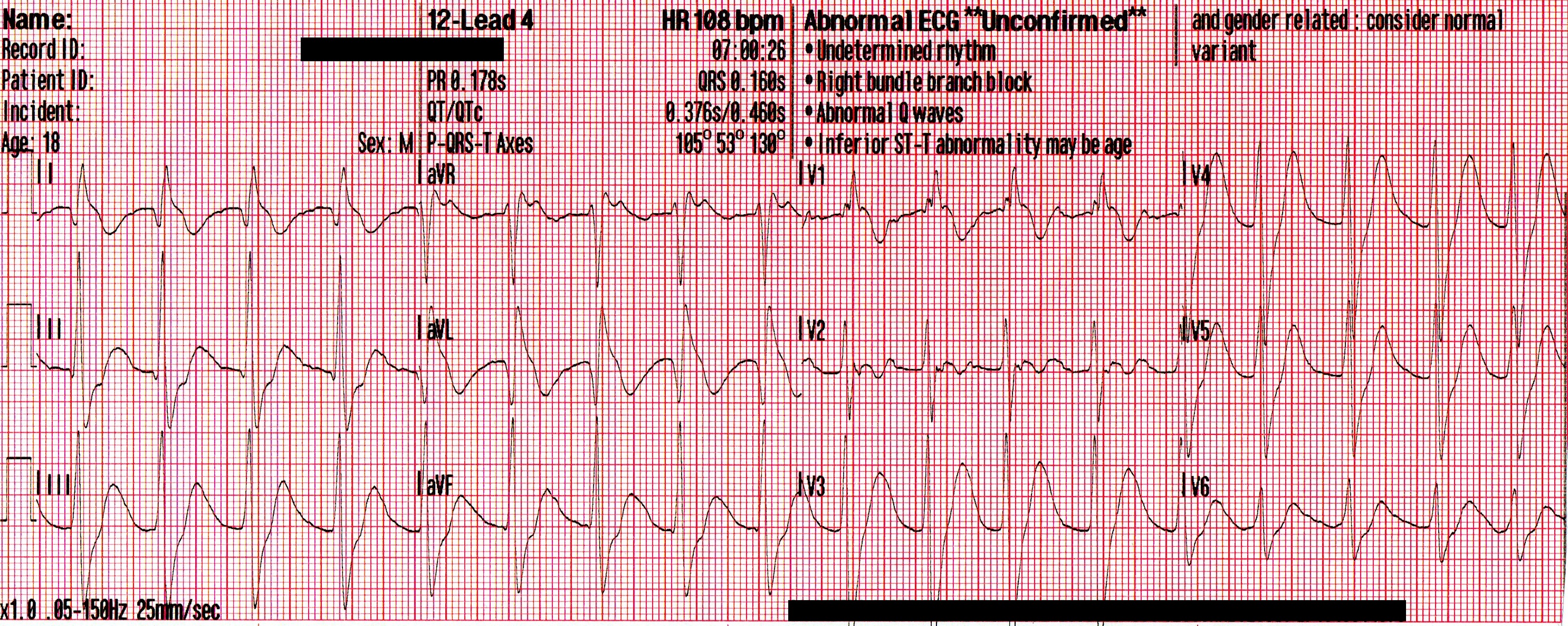
Prior to vasopressors being administered a change is noted on the cardiac monitor and another 12 lead ECG is obtained.
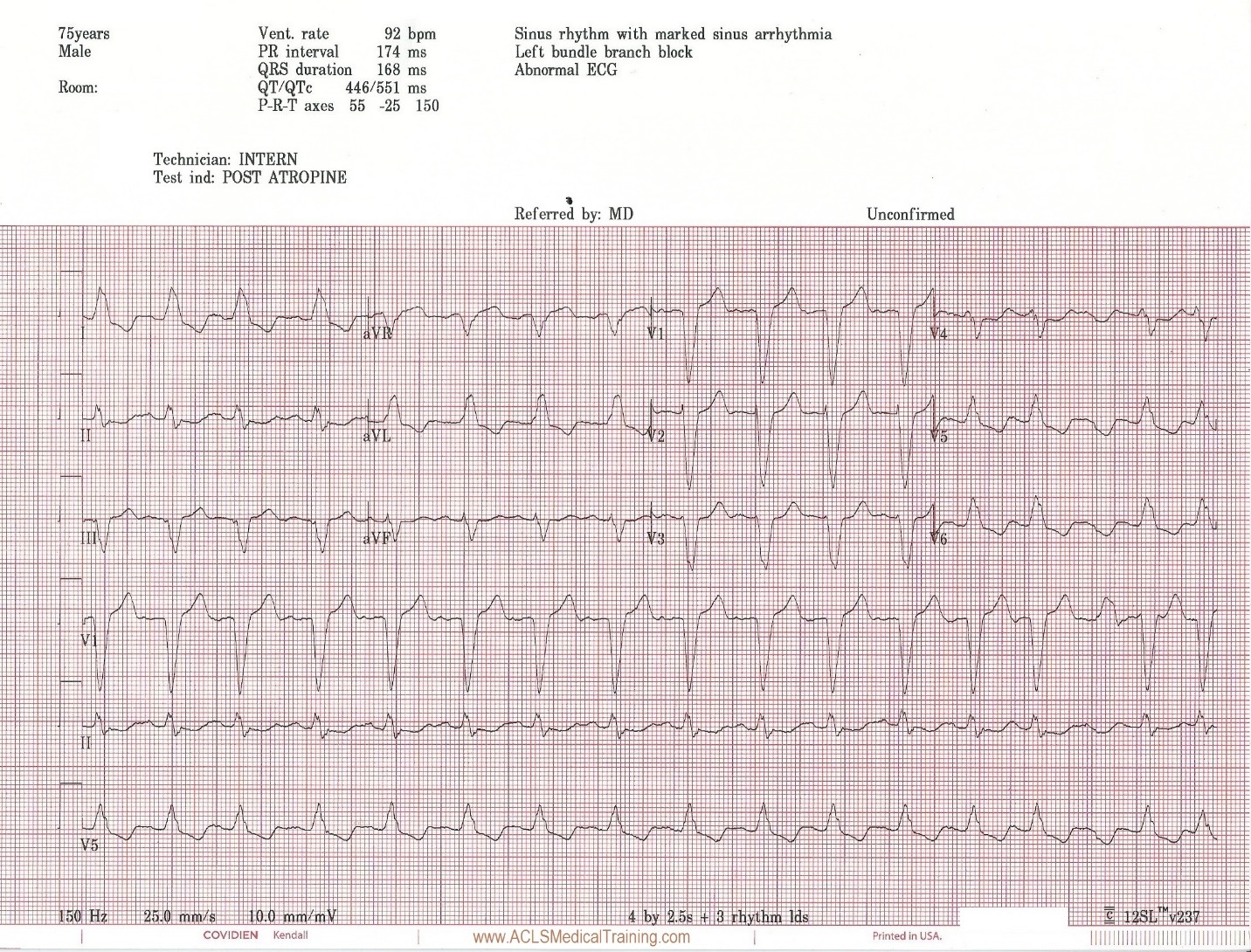
The heart rate is now 92. There is left bundle branch block which is consistent with the patient’s known medical history. At first glance this appears to be sinus rhythm although the last 3 cardiac cycles make the exact rhythm uncertain.
The patient now reports he is feeling better. His skin color improves and his blood pressure normalizes.
The patient was taken to cardiac cath lab for angiography and a permanent pacemaker. The procedure was successful and he was placed in the cardiac step-down unit for further observation.
Discussion
Atropine is an anticholinergic drug – also known as a parasympatholytic – which means that it counteracts increased vagal tone by binding to cardiac muscarinic receptors, which can improve sinus, atrial, and AV-nodal conduction. However, it is not believed to have a direct affect on rhythms of ventricular origin.
The 2010 AHA ECC Guidelines caution:
“Avoid relying on atropine in type II second-degree or third-degree AV block or in patients with third-degree AV block with a new wide-QRS complex where the location of block is likely to be in non-nodal tissue (such as in the bundle of His or more distal conduction system). These bradyarrhythmias are not likely to be responsive to reversal of cholinergic effects by atropine and are preferably treated with TCP…”
There is a caveat regarding TCP:
“TCP is, at best, a temporizing measure. TCP is painful in conscious patients, and, whether effective or not (achieving inconsistent capture), the patient should be prepared for transvenous pacing and expert consultation should be obtained. It is reasonable for healthcare providers to initiate TCP in unstable patients who do not respond to atropine. Immediate pacing might be considered in unstable patients with high-degree AV block when IV access is not available. If the patient does not respond to drugs or TCP, transvenous pacing is probably indicated.”
In this case transcutaneous pacing was not successful but the milliamperes were not increased beyond 90 milliamperes. On the plus side, the clinicians who were caring for this patient realized that they had not achieved capture, which is not always the case!
Fortunately, the patient spontaneously converted into a perfusing rhythm. The atropine may have contributed to the termination of this 3rd degree AV block, it may have been related to sympathetic stimulation from attempted transcutaneous pacing, or it could be a coincidence. These types of scenarios are susceptible to the “post hoc ergo propter hoc” fallacy (after this, therefore because of this).
A common error when treating patients with bradycardia is a rush to drug or electrical therapy prior to identifying reversible causes. Remember, Hs and Ts aren’t just for asystole and PEA!
Most importantly, hypoxemia should be rapidly identified and treated, but other conditions like hyperkalemia can also cause bradycardia. There is little to lose and much to gain from giving a patient calcium gluconate or calcium chloride prior to pacing. The quote Stephen Smith, M.D.: “The treatment [of hyperkalemia with calcium] is benign and cheap. How many life-threatening diseases can you treat benignly and cheaply?”
The definitive care for this patient was a permanent pacemaker.
Further reading: ACLS Bradycardia Algorithm
References
Neumar R, Otto C, Link M et al. Part 8: Adult Advanced Cardiovascular Life Support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18_suppl_3):S729-S767. doi:10.1161/circulationaha.110.970988.
Abrich V, Le R, Friedman P, et al. Aggressive Management of Bradycardia is Associated With Improved Clinical Outcomes and Shorter Length of Stay: A Comparison of Two Academic Centers. Circulation. 2016;134:A20838
EMCrit Podcast 42: A phD in EKG with Steve Smith. EMCrit Podcast. 2011. Available at: https://emcrit.org/emcrit/phd-in-ekg/. Accessed November 26, 2017.